ngwaahịa
gbasara anyị
E hiwere Micomme Medical Technology Development Co., Ltd na Changsha wee banye n'ihu ọha na-abanye n'ọhịa ikuku ventilashị na 2013. Micomme Medical lekwasịrị anya na ohuru maka ọgwụgwọ iku ume na ọrụ ọrịa iku ume na-adịghị ala ala, anyị na-agba mbọ ka ọ bụrụ ihe ngwọta ikuku na-adịghị emetụta ya. soplaya na China.
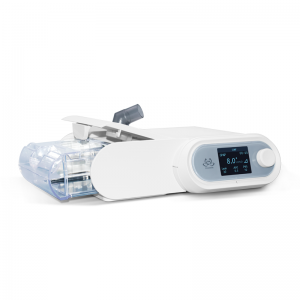
Pọtụfoliyo Micomme na-agụnye ikuku ikuku na-adịghị emerụ ahụ, nnukwu ikuku imi na-asọpụta, ikuku ikuku na-adịghị eme ihe n'ụlọ, ihe oriri, disinfector nke TUV CE kwadoro.Ọ bụghị naanị na China, anyị na-enyekwa ngwaahịa na ọrụ anyị karịa mba 60 n'ụwa niile.
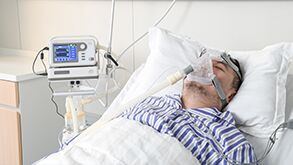
N'oge a na-alụ ọgụ megide COVID-19 na 2020, Micomme Medical chere oge gbara ọchịchịrị ya na ndị dọkịta na ndị nọọsụ anyị.Anyị na-anọ n'ihu ihu site na Wuhan, Beijing, Shanghai, Xinjiang ruo Italie, France ma ọ bụ Spain.Anyị na-emezu nkwa na ibu ọrụ anyị, anyị na-emeziwanye onwe anyị n'ịnọgidesi ike.Anyị na-eche banyere gị, site na ume ọ bụla!
Amụma anyị
Ahụike na ntụkwasị obi
Ọhụụ anyị
Jiri amamihe jeere obodo ozi.
Uru anyị
Nkà ihe ọmụma, ihe ọmụma, ọhụrụ, ịhụnanya
Mmụọ anyị
Ntachi obi
Ntachi obi bụ ihe nzuzo anyị nke imeri ihe isi ike.
Àgwà anyị
Ntachi obi na ọganihu
Standardkpụrụ anyị
Usoro dị elu, ọrụ zuru oke
Akwụkwọ akụkọ anyị, ozi kachasị ọhụrụ gbasara ngwaahịa anyị, ozi na onyinye pụrụ iche.
Pịa maka akwụkwọ ntuziaka-

Ogo
Na-edobe ịdịmma mgbe niile na mbụ ma na-elekọtakwa ngwaahịa ngwaahịa nke usoro ọ bụla.Anyị gafere asambodo usoro njikwa mma nke ISO9001 na sistemụ akụrụngwa ahụike nke ISO13485.
-

Asambodo
TÜV SÜD, ụlọ ọrụ mgbasa ozi German ama ama, nyere asambodo CE, nke na-egosi na ndị ọchịchị mba ụwa amatala teknụzụ ngwaahịa ngwaahịa na usoro ịdị mma nke ụlọ ọrụ ahụ.Anyị enwetala ndebanye aha na asambodo nke NMPA (CFDA na China) nke ọma.
-

Onye nrụpụta
Ọkachamara emeputa ngwaọrụ ikuku ikuku na-adịghị emebi emebi ihe fọrọ nke nta ka ọ bụrụ afọ 8.Ụlọ ọrụ anyị dị na Changsha, China.

ngwa
-
 2197
2197 Ngwa ụlọ ọgwụ ụlọ ọgwụ
-
 92
92 Patent nke mba
-
 39
39 Ọrụ nyocha sayensị
-
 52
52 Mmekọrịta na mba na mpaghara